Molecular Recognition in Drug Binding
Molecular recognition governs drug-target interactions, where pharmaceutical compounds bind specifically to biological targets, typically proteins. This specificity arises from precise molecular complementarity. Understanding these interactions enables design of highly selective drugs that minimize off-target effects. The spatial arrangement of functional groups critically influences binding efficiency.
Binding affinity results from multiple interaction types: hydrogen bonds, van der Waals forces, hydrophobic effects, and electrostatic attractions. These interactions collectively determine binding complex stability.
Drug-Target Interactions and Mechanisms
Drug-target interactions occur through various mechanisms including competitive and non-competitive inhibition. Comprehending these mechanisms is vital for therapeutic development and side-effect prediction.
The target's binding site geometry, determined by amino acid arrangement, dictates interaction specificity. This specificity ensures preferential target binding over other biological molecules.
Factors Affecting Drug Binding Affinity
Drug binding affinity depends on multiple factors: molecular properties (size, shape, charge), binding environment (pH, ionic strength), and competing molecules. Protein conformational changes also significantly influence interaction dynamics. These parameters must be carefully considered during drug design.
Computational Modeling of Drug-Target Interactions
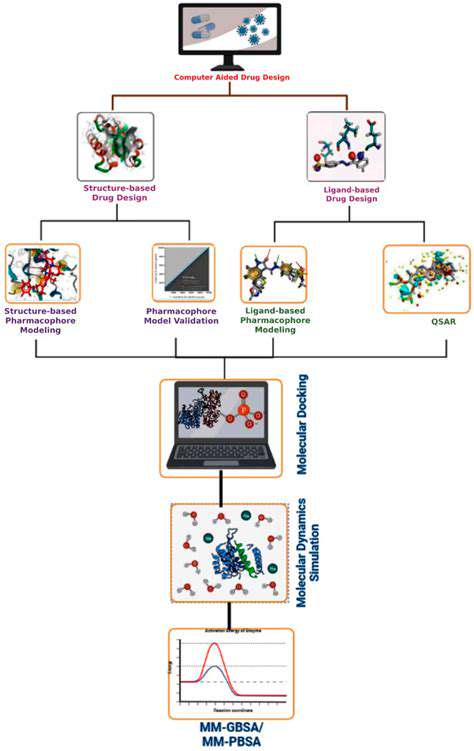
Computational approaches revolutionize drug-target interaction studies. Techniques like molecular docking and dynamics simulations predict binding modes and affinities. These methods accelerate discovery by identifying promising candidates and optimizing their binding properties.
Atomic-level simulations provide detailed binding insights, enabling design of safer, more effective drugs while predicting potential adverse interactions.
Drug Resistance and Molecular Interactions
Drug resistance often stems from target protein mutations that reduce drug affinity. Understanding resistance mechanisms is crucial for developing effective counterstrategies. Current approaches include designing multi-target drugs and compounds that bypass resistance mechanisms.
Predicting Drug Properties and Optimizing Design
Understanding Molecular Interactions
Quantum simulations analyze atomic-level drug candidate interactions by examining electron distributions and energy states. This reveals bonding patterns and molecular geometries critical for predicting biological behavior.
Detailed modeling of molecular forces enables design of selective drugs that target specific pathways while minimizing side effects.
Predicting Binding Affinity
Quantum methods predict drug-protein binding affinities by modeling atomic interactions including van der Waals forces and hydrogen bonding. Accurate predictions streamline drug candidate selection.
Optimizing Drug Design
Simulations enable structural optimization to enhance binding properties. Systematic evaluation of modifications facilitates development of compounds with improved therapeutic profiles.
Exploring ADMET Properties
Quantum methods predict absorption, distribution, metabolism, excretion, and toxicity characteristics by modeling biological interactions. Early identification of potential issues increases clinical trial success rates.
Investigating Reaction Mechanisms
Simulations elucidate drug metabolism pathways and protein interactions, providing critical data for efficacy and safety optimization.
Simulating Molecular Dynamics
Molecular dynamics simulations visualize drug molecule behavior over time, offering insights into biological system interactions and movement patterns.
Computational Screening of Libraries
Virtual screening of molecular libraries identifies promising candidates with desirable properties, significantly accelerating early-stage discovery processes.
From Simulation to Synthesis: Accelerating the Drug Development Pipeline
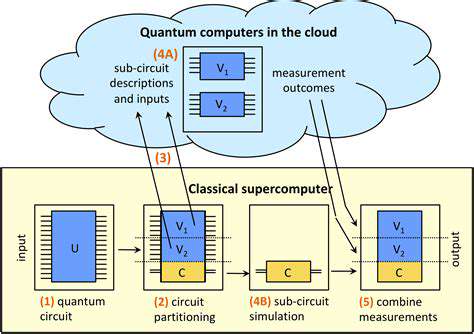
Harnessing Quantum Computing for Drug Discovery
Quantum computing revolutionizes drug discovery through enhanced molecular modeling capabilities. This technology enables precise prediction of drug-target interactions, significantly reducing development timelines.
Quantum methods explore chemical spaces beyond classical computational limits, facilitating identification of optimal molecular configurations.
Simulating Molecular Interactions with Unprecedented Accuracy
Quantum simulations surpass traditional approximations, providing nuanced understanding of molecular behaviors. This precision enables development of highly targeted therapeutics with minimized side effects.
Accelerating Lead Identification and Optimization
Quantum methods rapidly screen molecular libraries and refine lead compounds, substantially shortening the candidate selection process.
From Theory to Practice: Bridging the Gap Between Simulation and Synthesis
Quantum computing facilitates translation of theoretical insights into practical molecular design, accelerating the entire development pipeline.
The Future of Quantum-Enhanced Drug Discovery
Quantum technology promises to transform pharmaceutical research through accelerated discovery and improved therapeutic efficacy. Continued advancements will enable more personalized medicine approaches.
Advanced Techniques and Future Directions

Advanced Machine Learning Algorithms
Deep learning techniques analyze complex datasets to identify subtle patterns imperceptible to traditional methods. These models excel in pattern recognition and autonomous decision-making tasks.
Reinforcement learning adapts strategies through environmental feedback, proving particularly effective in dynamic systems like robotics.
Data Augmentation and Feature Engineering
Data expansion techniques enhance model training when datasets are limited. Feature extraction converts raw data into meaningful model inputs, often requiring specialized domain knowledge.
Explainable AI (XAI) and Ethical Considerations
As models grow more complex, interpretability becomes crucial, especially in sensitive applications. Ethical implementation requires careful attention to potential biases in training data and algorithms.
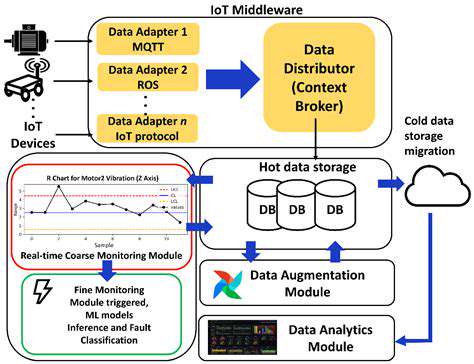
Edge Computing and Real-time Applications
Localized data processing through edge computing enables rapid response in time-sensitive applications. This approach proves vital for autonomous systems requiring instantaneous decision-making.